PCl3 Lewis Structure in Four Simple Steps What's Insight
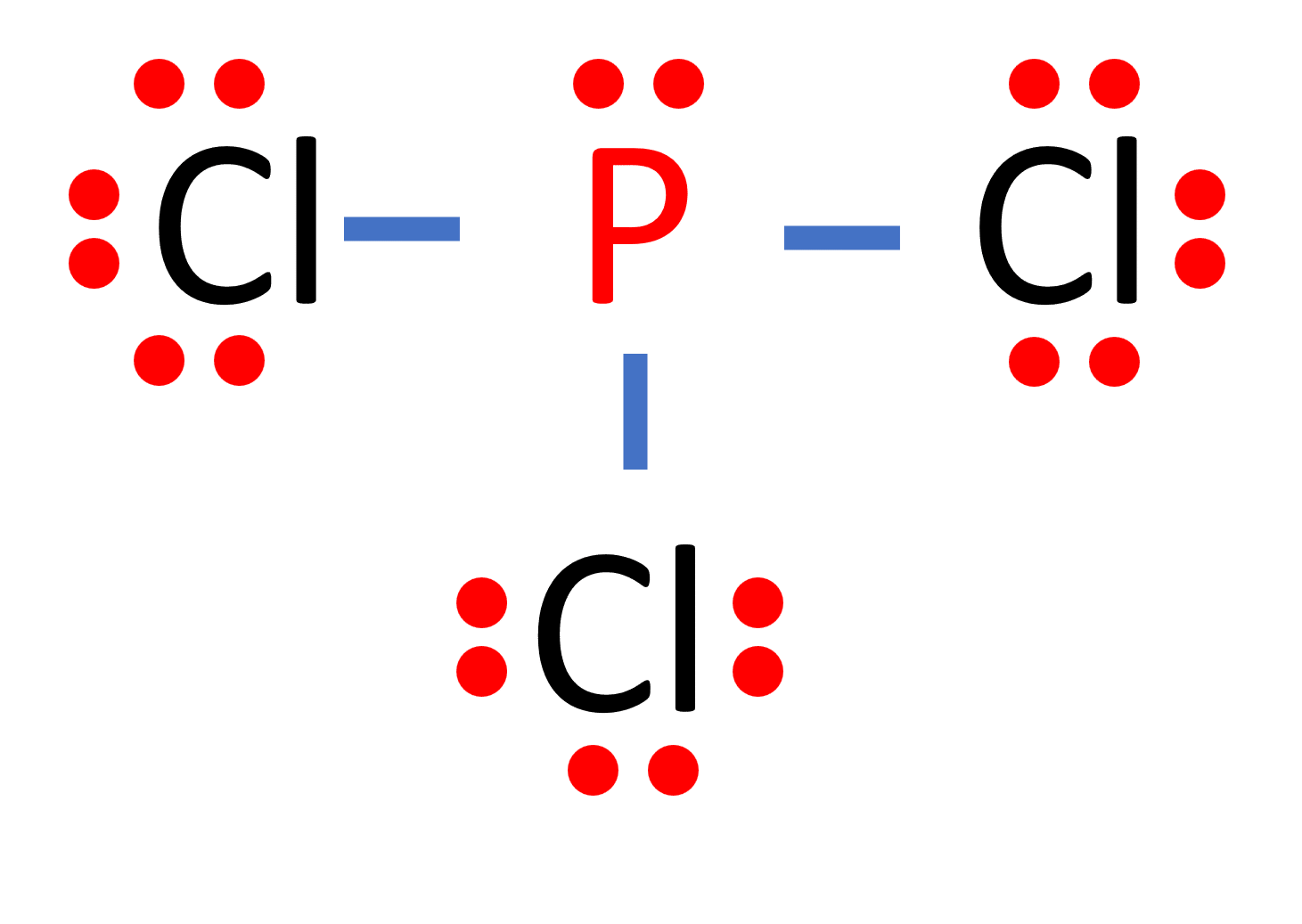
PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.

How to draw PCl3 Lewis Structure? Science Education and Tutorials
The Lewis structure of PCl3 shows that phosphorus (P) is the central atom bonded to three chlorine (Cl) atoms. The central phosphorus atom has a lone pair of electrons and forms three single bonds with chlorine atoms. The Lewis structure helps in understanding the molecular geometry and chemical properties of PCl3.
35 Lewis Dot Diagram For Pcl3 Wiring Diagram List
I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle.

PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram
Steps of drawing PCl3 lewis structure Step 1: Find the total valence electrons in PCl3 molecule. In order to find the total valence electrons in PCl3 molecule, first of all you should know the valence electrons present in phosphorus atom as well as chlorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.).

PCl3 Lewis StructureLewis Structure of PCl3 (Phosphorus Trichloride
3.1: Lewis Structures. Chemical bond refers to the forces holding atoms together to form molecules and solids. This force is of an electric nature, and the attraction between electrons of one atom to the nucleus of another atom contributes to what is known as chemical bonds.

PCl3 Molecular Geometry,Shape and Bond Angles (Phosphorous Trichloride
PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram. Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. This liquid can be colorless as well. PCl3 is a toxic liquid with an unpleasant smell. The molar mass of this compound is 137.33 g/mol.
PCl3 Molecular Geometry / Shape and Bond Angles YouTube
Phosphorus pentachloride is the chemical compound with the formula PCl 5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl 3 and POCl 3. PCl 5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride .

Lewis Dot Diagram For Pcl3 General Wiring Diagram
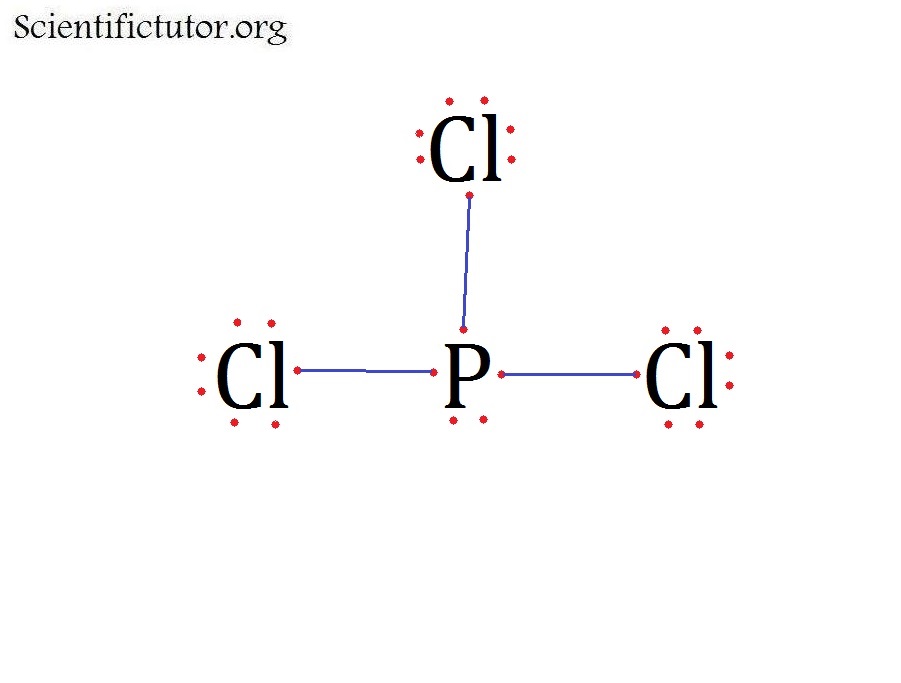
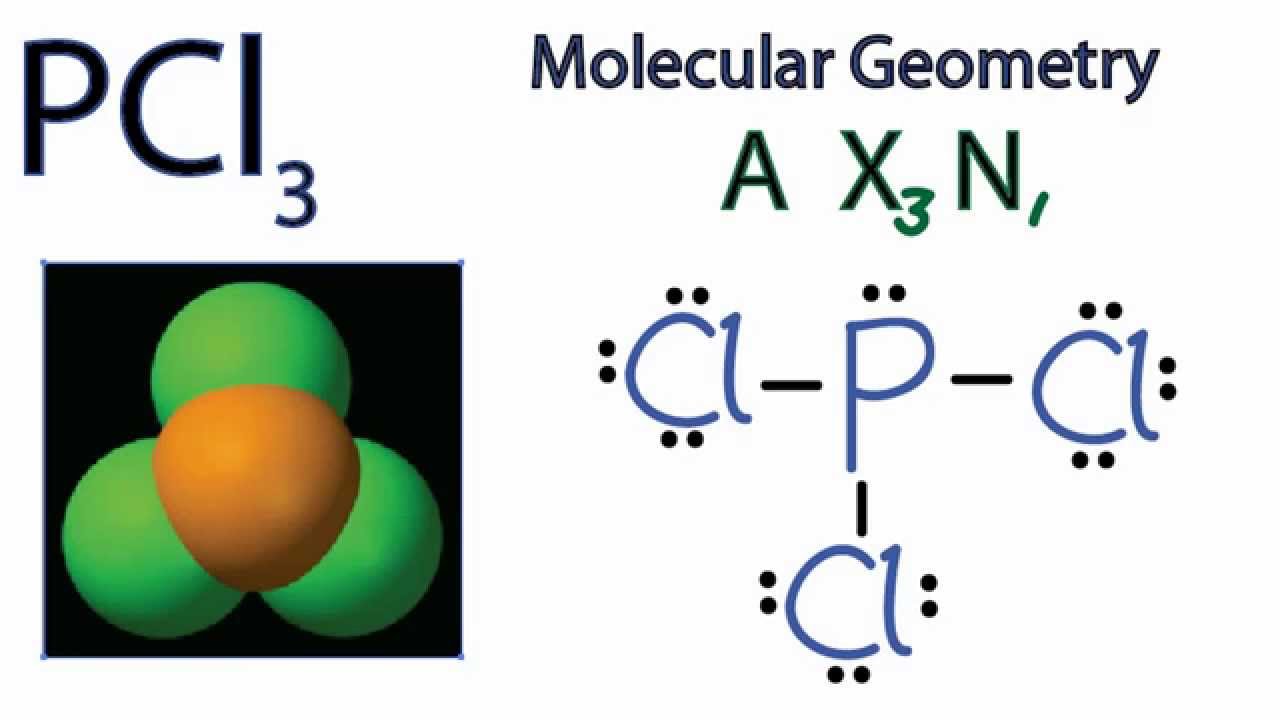
The molecular geometry or shape of PCl 3 is a Trigonal pyramid. The electron geometry of PCl 3 is Tetrahedral, as its central atom, is surrounded by the 4 regions of electron density. In the PCl 3 Lewis dot structure, a total of 10 lone pairs and 3 bond pairs are present. The hybridization of phosphorous in PCl 3 is sp 3.

PCL3 Molecular Electron Geometry, Lewis Structure, Bond Angles and
A quick explanation of the molecular geometry of PCl3 including a description of the PCl3 bond angles.Looking at the PCl3 Lewis structure we can see that the.

Lewis Dot Diagram For Pcl3 General Wiring Diagram
Step #1: Calculate the total number of valence electrons. Here, the given molecule is PCl3 (phosphorus trichloride). In order to draw the lewis structure of PCl3, first of all you have to find the total number of valence electrons present in the PCl3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an.
Lewis Dot Diagram For Pcl3 General Wiring Diagram
Phosphorus trichloride is the precursor to organophosphorus compounds. It reacts with phenol to give triphenyl phosphite : 3 PhOH + PCl3 → P (OPh)3 + 3 HCl (Ph = C6H5) Alcohols such as ethanol react similarly in the presence of a base such as a tertiary amine: [9] PCl3 + 3 EtOH + 3 R3N → P (OEt)3 + 3 R3NH+Cl−.

So far, we’ve used 26 of the PCl3 Lewis structure’s total 26 outermost
PCl 3; NOF; Solution. The first step is to draw the Lewis structure of the molecule. For \(\ce{PCl3}\), the electron dot diagram is as follows: The lone electron pairs on the Cl atoms are omitted for clarity. The P atom has four electron groups with three of them bonded to surrounding atoms, so the molecular shape is trigonal pyramidal.

lewis structure and molecular geometry of PCl3? OpenStudy
Example \(\PageIndex{1}\) Draw the Lewis structures of CH 4, PCl 3, CO 2, and HCN. Solution. Step 1: Add the valence electrons of all the molecules' atoms:. CH 4 has 4 valence electrons in C, and 1 in each of the four H: = 4 + 1x4 = 8 valence electrons; PCl 3 has 5 valence electros in P and 7 in each of the three Cl: = 5 + 7x3 = 26 valence electrons; CO 2 has 4 valence electrons in C and 6 in.
[Solved] what is the lewis structure, electron geometry, molecular
PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and Hybridization. Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries.

Structure Phosphorous Trichloride (PCL3), Grade Standard Technical
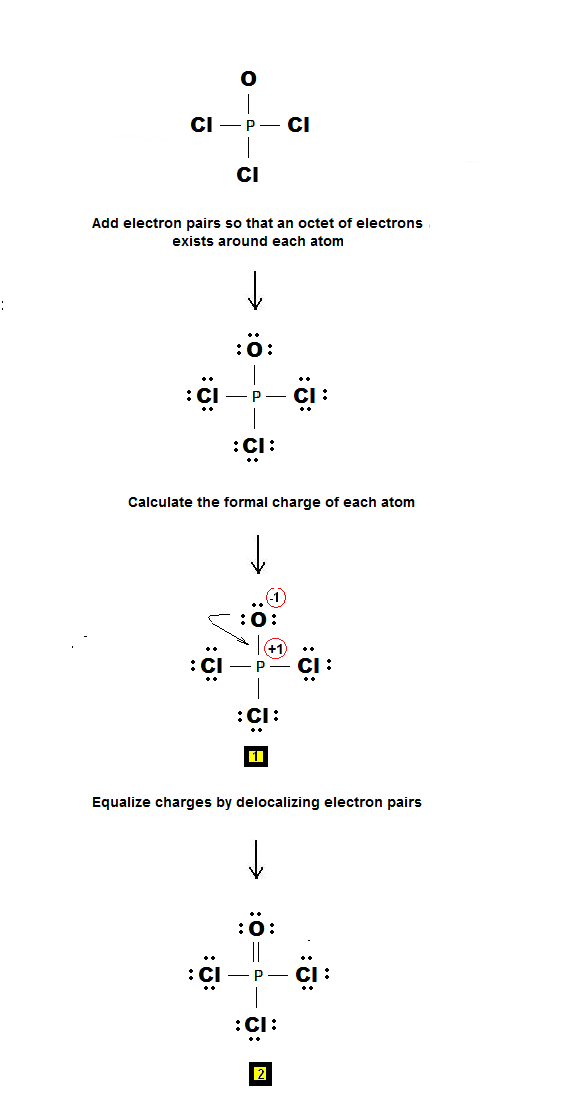
Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get.
New Vsepr Pcl3 Molecular Geometry PNG GM
Check me out: http://www.chemistnate.com